How to Find Number of Protons
Force definition physical power or strength possessed by a living being. Find the Number of Protons.
The lithium atom contains about eleven isotopes.

. In cosmology recombination refers to the epoch during which charged electrons and protons first became bound to form electrically neutral hydrogen atomsRecombination occurred about 370000 years after the Big Bang at a redshift of z 1100The word recombination is misleading since the Big Bang theory doesnt posit that protons and electrons had been. Of protons 17 of neutrons 37 17 20 of electrons 17 0 17 of protons 16 the atomic number is not given but can be found on the. Of protons atomic number of neutrons mass number atomic number of electrons atomic number charge.
Build an atom out of protons neutrons and electrons and see how the element charge and mass change. Among the isotopes 6 Li and 7 Li are stable and are formed naturally. The results of his experiments show that each element has a unique integer equal to the number of positive charges in the nucleus of that element.
For example an oxygen atom possesses nine neutrons and eight protons. Scientist Henry Gwynn Jefferies Mosle examined the X-ray spectrum of various elements in 1913-to 1914. The atomic number from the atomic mass will give you the calculated number of neutrons in the atom.
For example in a sodium atom there are 11 electrons and 11 protons. A n Z. Then play a game to test your ideas.
Such as 3 Li 4 Li 5 Li 6 Li 7 Li 8 Li 9 Li 10 Li 11 Li 12 Li and 13 Li. How to find the Atomic Mass. The negative charge of each electron is found by experiment to have the same magnitude which is also equal to that of the positive charge of each proton.
There are 275 isotopes of the 81 stable elements in addition to over 800 radioactive isotopes and every element has known isotopic forms. Since the vast majority of an atoms mass is made up of its protons and neutrons subtracting the number of protons ie. It is defined as where n q is the number of quarks and n q is the number of antiquarks.
The easiest way to find the atomic mass of an element is to look on the periodic table. Atomic mass number of protons number of neutrons. No matter how many electrons or neutrons an atom has the element is defined by its number of protons.
In particle physics the baryon number is a strictly conserved additive quantum number of a system. The atomic number of an atom is equal to the number of protons in the nucleus of an atom or the number of electrons in an electrically neutral atom. Subtract the atomic number from the atomic mass.
The numbers after the decimal point represent the usually very small mass of the. The number of protons in an isotope atom does not change but the number of neutrons does. He used all his force in opening the window.
Thus we put the values in the formula to find atomic mass such as. Great lets apply the rules to some examples. To find the number of neutrons you will need to subtract the atomic number from the atomic mass.
Many fundamental or subatomic particles of matter have the property of electric charge. Lets also look at the next two alkanes propane and butane before trying to find some patterns for determining the number of NMR signals a little easier. A weighted average of the number of neutrons and protons present for all isotopes.
How to find the Atomic Number. In other words the period number indicates the number of energy levels or energy orbit of an atom. How to easily find the number of electrons protons and neutrons in an oxygen atom.
It is the average weight of an atom or molecule. Atomic number Number of protons. Isotope definition any of two or more forms of a chemical element having the same number of protons in the nucleus or the same atomic number but having different numbers of neutrons in the nucleus or different atomic weights.
Atomic mass is related to the number of neutrons and protons present in a particular nucleus of an element. Subtract the atomic number from the atomic mass. Number of protons present in an atom.
The atomic number is usually the number of protons present in the nucleus of an element that you can also determine using this best atomic number calculator. For our boron example 11 atomic mass 5 atomic number 6 neutrons. Thus the atomic number of Na atom number of electrons number of protons 11.
Here A is the mass number n is the number of neutrons and Z is the atomic number number of protons. Baryons three quarks have a baryon number of 1 mesons one quark one antiquark have a baryon number of 0 and antibaryons three antiquarks have a baryon. For example electrons have negative charge and protons have positive charge but neutrons have zero charge.
The atomic number of an element is simply the number of protons in. FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation. Each element is defined by the number of protons found in each of its atoms.
A 9 8. For example 1st period indicates that these elements possess 1 energy shell or energy orbit. Remember that the atomic number is the same as the number of protons which you have already identified.
The worlds largest and most powerful particle accelerator has restarted after a break of more than three years for maintenance consolidation and upgrade work. Propane and butane give two signalsOne because the protons of the CH 2 group are different from those in the CH 3 group and the other because despite having four carbon atoms the molecule is a combination of two identical CH. The period number on the Periodic table tells you the total number of orbits that the atom will have.
In fact its actually possible to have an atom consisting of only a proton ionized hydrogen. A pure substance that cannot be broken down into a simpler substance by chemical means. Build an Atom - PhET.
Carbon has 6 protons in its nucleus making it also the sixth element in the periodic table. What does the period number tell you. Today 22 April at 1216 CEST two beams of protons circulated in opposite directions around the Large Hadron Colliders 27-kilometre ring at their injection energy of 450 billion electronvolts 450 GeV.
The atomic number of an element is found through the number of protons present in the nucleus.
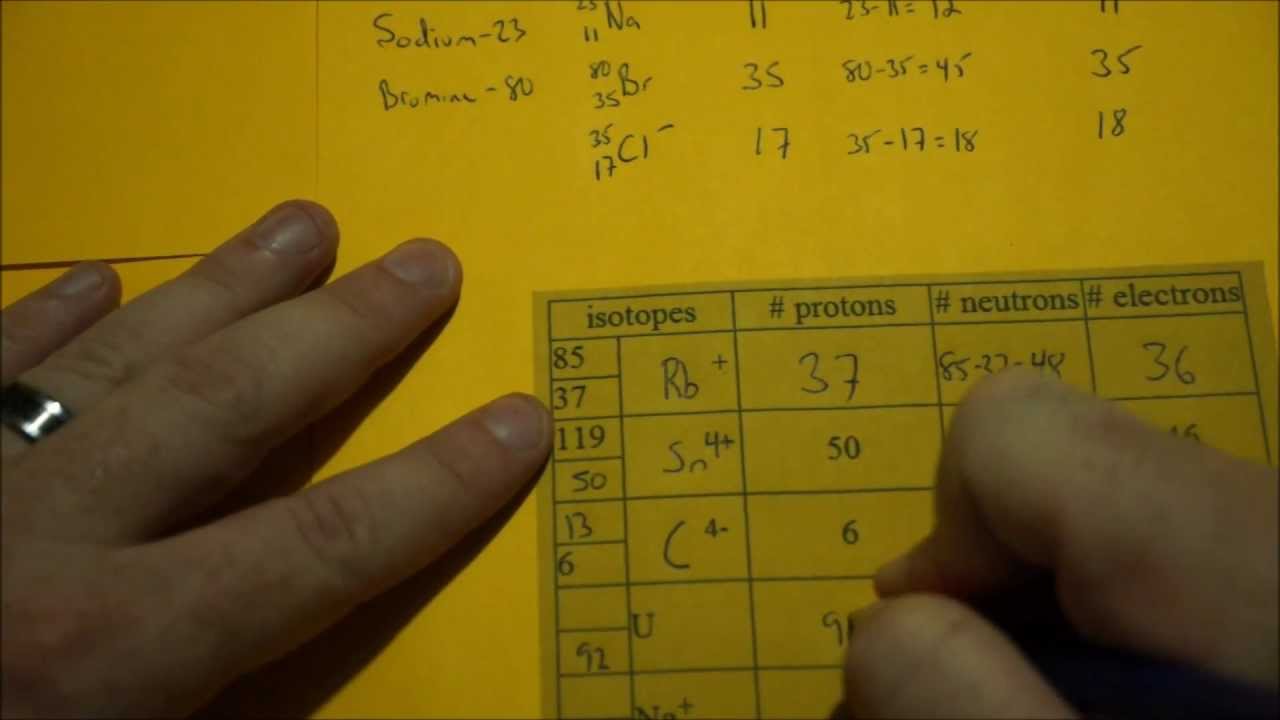
Determining Protons Neutrons And Electrons Atoms And Ions Protons Neutrons Electrons

Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic Proton Neutron Electron Protons Neutrons
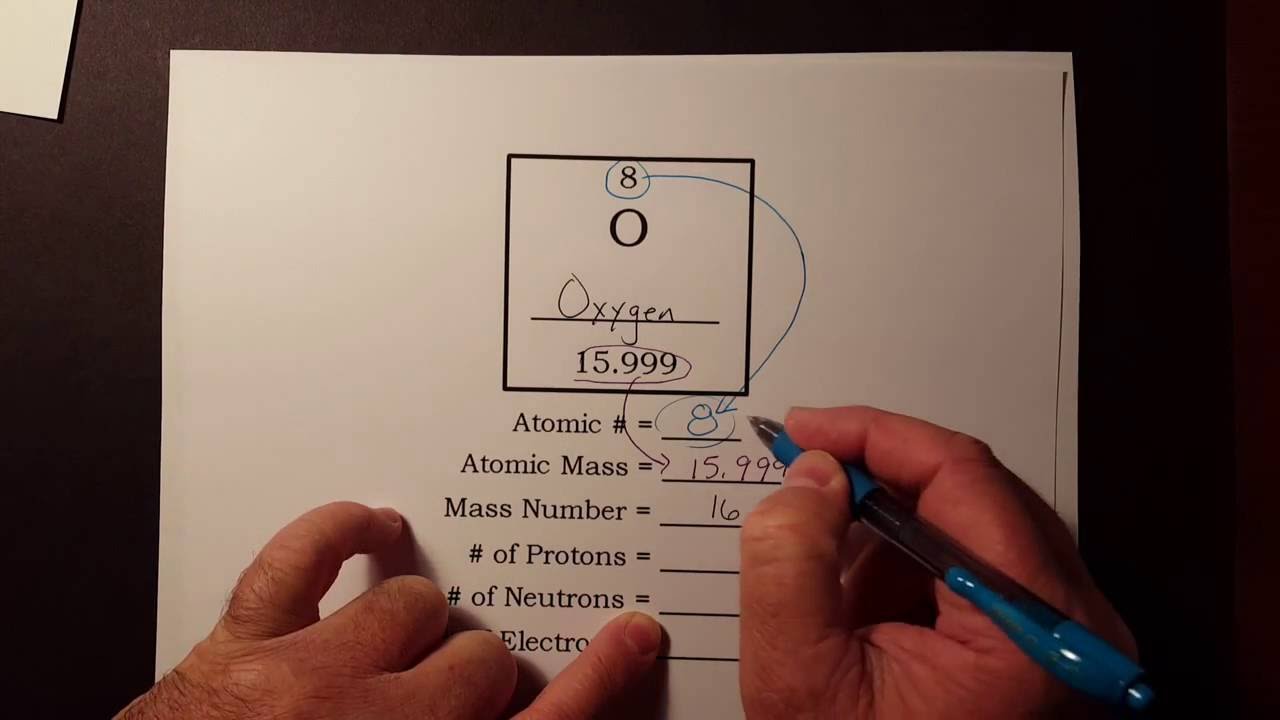
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Protons Proton Neutron Electron

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets

No comments for "How to Find Number of Protons"
Post a Comment